
Chemistry, 03.07.2019 20:30 TheGingerDevl7762
Ascientist measures the standard enthalpy change for the following reaction to be 595.8 kj : 2h2o(l)2h2(g) + o2(g) based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of h2o(l) is kj/mol.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1

Chemistry, 22.06.2019 20:00
Carbon-14 undergoes radioactive decay in the reaction above. determine the type of radiation emitted in this reaction and describe what is happening to the nucleus during this reaction.
Answers: 2

Chemistry, 22.06.2019 20:30
Which states of matter have particles that move independently of one another with very little attraction?
Answers: 1

You know the right answer?
Ascientist measures the standard enthalpy change for the following reaction to be 595.8 kj : 2h2o(l...
Questions


Mathematics, 02.03.2021 19:20



Mathematics, 02.03.2021 19:20

Mathematics, 02.03.2021 19:20

Mathematics, 02.03.2021 19:20

English, 02.03.2021 19:20

Mathematics, 02.03.2021 19:20

Mathematics, 02.03.2021 19:20


Mathematics, 02.03.2021 19:20




Mathematics, 02.03.2021 19:20


Mathematics, 02.03.2021 19:20


Mathematics, 02.03.2021 19:20

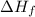 for
for 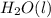 in the reaction is 297.9 kJ/mol.
in the reaction is 297.9 kJ/mol.![\Delta H_{rxn}=\sum [n\times \Delta H_f(product)]-\sum [n\times \Delta H_f(reactant)]](/tpl/images/0047/6370/db29b.png)
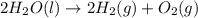
![\Delta H_{rxn}=[(2\times \Delta H_f_{(H_2)})+(1\times \Delta H_f_{(O_2)})]-[(2\times \Delta H_f_{(H_2O)})]](/tpl/images/0047/6370/b664a.png)
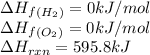
![595.8=[(2\times (0))+(1\times (0))]-[2\times (\Delta H_f_{H_2O})]\\\\\Delta H_f_{H_2O}=297.9kJ/mol](/tpl/images/0047/6370/81985.png)


