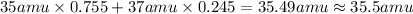The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chlorine atoms have 18 neutrons and 17 protons, and the other 24.5% have 20 neutrons and 17 protons. using the isotopic composition provided, calculate the average atomic mass of chlorine. round your answer to the tenths place.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 15:50
Where are chemicals found at work? a. only in cleaning products b. only in carpets and paint c. in every area of work d. only in food preparation submit
Answers: 1

Chemistry, 21.06.2019 17:30
One mole of zinc has a mass of 65.4 grams. approximately how many atoms of zinc are present in one mole of zinc? 32 × 1023 atoms 6 × 1023 atoms 66 atoms 65 atoms
Answers: 1


Chemistry, 22.06.2019 10:30
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1
You know the right answer?
The element chlorine (cl) has two isotopes: chlorine‑35 and chlorine‑37. approximately 75.5% of chl...
Questions


Mathematics, 24.01.2020 12:31





Health, 24.01.2020 12:31

History, 24.01.2020 12:31


Mathematics, 24.01.2020 12:31




Mathematics, 24.01.2020 12:31

Mathematics, 24.01.2020 12:31


History, 24.01.2020 12:31



Geography, 24.01.2020 12:31