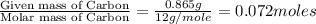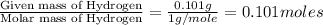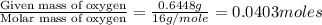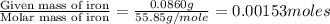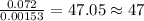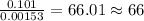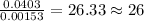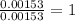Chemistry, 03.07.2019 21:20 delanieloya
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of co2 and 0.90829 g of h2o were produced. in a separate experiment to determine the mass percent of iron, 0.5446 g of the compound yielded 0.1230 g of fe2o3. what is the empirical formula of the compound?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 19:10
Astudent completes a titration by adding 12.0 milliliters of naoh(aq) of unknown concentration to 16.0 milliliters of 0.15 m hcl(aq). what is the molar concentration of the naoh(aq)? 1)5.0 m 2)0.20 m 3)0.11 m 4)1.1 m
Answers: 1

Chemistry, 22.06.2019 19:30
Which one of the following substances would be the most soluble in ccl4? na2so4 h2o ch3ch2ch2ch2oh c4h10 hi
Answers: 1

Chemistry, 22.06.2019 20:30
Citric acid has a ph between 1 and 3. it is considered to be aa. weak acidb. weak basec. strong based. strong acid
Answers: 2

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1
You know the right answer?
When 1.6968 g of an organic iron compound containing fe, c, h, and o was burned in o2, 3.1737 g of c...
Questions


Computers and Technology, 18.03.2022 14:00

English, 18.03.2022 14:00




Mathematics, 18.03.2022 14:00

History, 18.03.2022 14:00



History, 18.03.2022 14:00



History, 18.03.2022 14:00

Mathematics, 18.03.2022 14:00

Mathematics, 18.03.2022 14:00

Mathematics, 18.03.2022 14:00



English, 18.03.2022 14:00

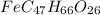
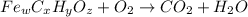
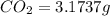
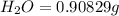
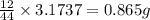 of carbon will be contained.
of carbon will be contained.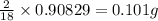 of hydrogen will be contained.
of hydrogen will be contained.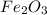 =
= 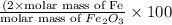
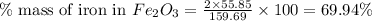
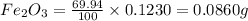 of iron.
of iron.