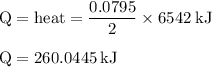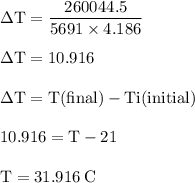Chemistry, 03.07.2019 21:20 tannerweberp5r8sg
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 ∘ c, what is the final temperature of the water?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 14:00
Displacement is the slope of a velocity vs. time graph a. true b. false
Answers: 1

Chemistry, 22.06.2019 16:00
Answer asap : ( a. how does mucus prevent the entry of pathogens? b. describe two ways white blood cells protect us from pathogens.
Answers: 1

Chemistry, 22.06.2019 20:30
The activation energy for the reaction no2(g)+co2(g)⟶no(g)+co(g) is ea = 300 kj/mol and the change in enthalpy for the reaction is δh = -100 kj/mol . what is the activation energy for the reverse reaction?
Answers: 3
You know the right answer?
The balanced combustion reaction for c6h6 is 2c6h6(l)+15o2(g)⟶12co2(g)+6h2o(l)+6 542 kj if 6.200 g c...
Questions



Physics, 05.12.2019 23:31


English, 05.12.2019 23:31

Social Studies, 05.12.2019 23:31

History, 05.12.2019 23:31

Chemistry, 05.12.2019 23:31


Biology, 05.12.2019 23:31


Mathematics, 05.12.2019 23:31


Mathematics, 05.12.2019 23:31



Chemistry, 06.12.2019 00:31


Mathematics, 06.12.2019 00:31