Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 15:50
How many moles of potassium hydroxide are needed to completely react with 2.94 moles of aluminum sulfate
Answers: 1

Chemistry, 22.06.2019 16:00
Which factor is likely to impact the possible number of compounds ?
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 20:00
The picture represents the process that produces most of the energy used by living organisms on earth. which process is represented in the picture? a) the magnetic attraction between two hydrogen nuclei. b) the fusion of hydrogen nuclei to produce a helium nucleus in the core of the sun. c) the fission of hydrogen nuclei to produce a helium nucleus in the core of the sun. d) the chemical reaction between hydrogen nuclei to produce a helium nucleus in earth's atmosphere.
Answers: 3
You know the right answer?
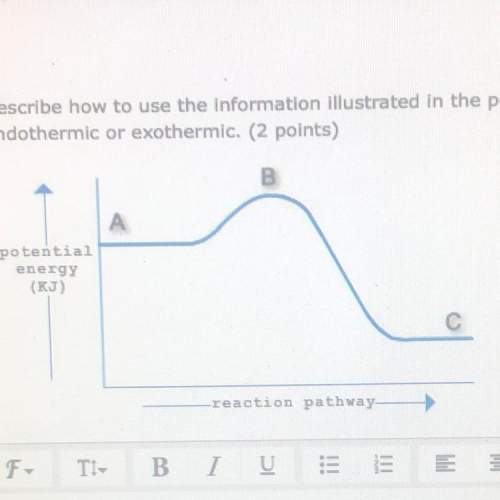
Describe how to use the information illustrated in the potential energy diagram below to determine t...
Questions




Biology, 28.09.2019 03:30

English, 28.09.2019 03:30


Health, 28.09.2019 03:30


Mathematics, 28.09.2019 03:30



Geography, 28.09.2019 03:30






Mathematics, 28.09.2019 03:30


Chemistry, 28.09.2019 03:30