
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydrochloric acid to a sample of chalk and measuring the volume of carbon dioxide produced caco3(aq) + 2hcl -> cacl2(aq) + co2(g) + h2o(g) excess hydrochloric acid was added to 0.5g chalk and 100cm3 of carbon dioxide gas was given produced at r. t.p calculate the percentage purity of calcium carbonate in sample of chalk

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 06:30
Over the last 90 years, scientists have added to the body of evidence supporting the big bang theory. what is the latest piece of evidence discovered in 2014?
Answers: 1

Chemistry, 22.06.2019 18:00
An object displaces 652 ml of water. the volume of the object is: 0.652 cm³ 6.52 cm³ 65.2 cm³ 652 cm³
Answers: 3

Chemistry, 22.06.2019 22:30
Akno3 solution containing 51 g of kno3 per 100.0 g of water is cooled from 40 ∘c to 0 ∘c. what will happen during cooling?
Answers: 3
You know the right answer?
Chalk is impure calcium carbonate. the amount of calcium carbonate present can be determined by hydr...
Questions

Chemistry, 17.12.2020 07:50

World Languages, 17.12.2020 07:50


History, 17.12.2020 07:50

Arts, 17.12.2020 07:50


Mathematics, 17.12.2020 07:50

History, 17.12.2020 07:50

Mathematics, 17.12.2020 07:50

World Languages, 17.12.2020 07:50

Mathematics, 17.12.2020 07:50


English, 17.12.2020 07:50

English, 17.12.2020 07:50


Computers and Technology, 17.12.2020 07:50

Mathematics, 17.12.2020 07:50

Health, 17.12.2020 07:50

Mathematics, 17.12.2020 07:50

History, 17.12.2020 07:50

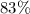 .
.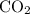 gas are released?
gas are released?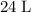 under room temperature and pressure (r.t.p,
under room temperature and pressure (r.t.p, 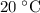 ,
, 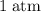 .) That's the same as
.) That's the same as 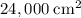 .
.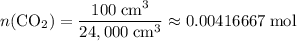 .
.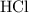 is in excess. How many moles of
is in excess. How many moles of 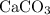 formula units will produce that
formula units will produce that 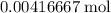 of
of 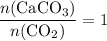 .
.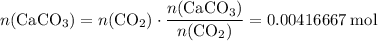 .
.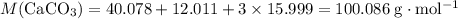 .
.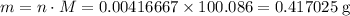 .
.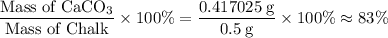 .
.

