
Chemistry, 05.07.2019 06:10 cupkakekawaii45
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system? so2(g) + no2(g) ⇌ so3(g) + no(g) a) the reaction will shift in the direction of products. b) the reaction will shift to decrease the pressure. c) no change will occur since so3 is not included in the equilibrium expression. d) the reaction will shift in the direction of reactants. e) the equilibrium constant will decrease.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:10
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2

Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 04:00
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2
You know the right answer?
Consider the following reaction at equilibrium. what effect will adding more so3 have on the system?...
Questions




Mathematics, 08.11.2019 19:31



History, 08.11.2019 19:31


Health, 08.11.2019 19:31

Mathematics, 08.11.2019 19:31


Mathematics, 08.11.2019 19:31


English, 08.11.2019 19:31





Chemistry, 08.11.2019 19:31

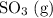 (analog to concentration in a solution.)
(analog to concentration in a solution.) 

