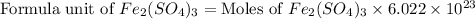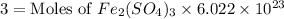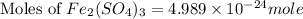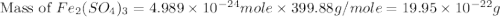The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the number of sulfur atoms in 1.75 mole of fe(so4)3
b) the mass in grams of 2.65 mol of fe(so4)3
c) the number of moles of fe(so4)3 in 3.45 grams of fe(so4)3.
d)the mass in grams of 3 formula unit of fe(so4)3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:00
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1

Chemistry, 22.06.2019 14:20
7. in the cycle, a virus integrates its dna into the host's dna, and its dna is replicated when the host dna is replicated. a. infectious b. retroviral c. lysogenic d.lytic
Answers: 1

Chemistry, 23.06.2019 02:30
Which of the four hypothetical substances you investigated would be most harmful to living organisms? 50 points!
Answers: 2

Chemistry, 23.06.2019 03:40
The following questions a24 - a26 relate to 100 ml of 0.0150 m solution of benzoic acid (c6h3cooh). ka(c6h3cooh) = 6.4 x 10^-5. what is the ph of the solution after the addition of 1 x 10^-3 moles of naoh? you may assume no volume change to the solution upon addition of the naoh.
Answers: 2
You know the right answer?
The chemical formula for ferric sulfate is fe(so4)3. determine the following:
a) the nu...
a) the nu...
Questions



Law, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30

Geography, 08.12.2021 05:30

English, 08.12.2021 05:30

Social Studies, 08.12.2021 05:30


Chemistry, 08.12.2021 05:30

Biology, 08.12.2021 05:30


History, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30


Mathematics, 08.12.2021 05:30



 .
.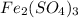 is, 1059.682 grams.
is, 1059.682 grams.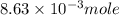
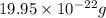
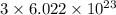 number of sulfur atoms.
number of sulfur atoms.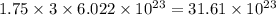 number of sulfur atoms.
number of sulfur atoms.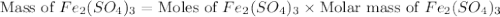
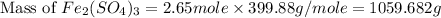
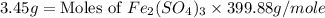
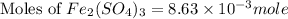
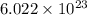 formula unit.
formula unit.