
Chemistry, 05.02.2020 12:51 acervantes29
A) what is the mass in grams of a sample of manganese (ii) phosphite containing 7.23 x 10^22 phosphite ions?
b) determine the percent composition (by mass) for each element in ammonium dichromate (nh4)2cr2o7
c) a compound with a molecular mass of 202.23g/mol was found to have the following mass percent composition: 95.02% carbon and 4.98% hydrogen. determine its:
*empirical formula
*molecular formula

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:00
2h2s + 3o2 2so2 + 2h2o which option gives the correct mole ratios? h2s: so2 = 2: 2 and o2: h2o = 3: 2 h2s: so2 = 2: 3 and o2: h2o = 3: 2 h2s: so2 = 4: 4 and o2: h2o = 5: 4 h2s: so2 = 4: 6 and o2: h2o = 4: 4
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

Chemistry, 22.06.2019 06:00
How much would the freezing point of water decrease if 4 mol of sugar were added to 1 kg of water(k=1.86 c/mol/kg for water and i=1 for sugar
Answers: 1

You know the right answer?
A) what is the mass in grams of a sample of manganese (ii) phosphite containing 7.23 x 10^22 phosphi...
Questions

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01



Mathematics, 19.07.2020 01:01





History, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01

Mathematics, 19.07.2020 01:01



Spanish, 19.07.2020 01:01


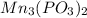

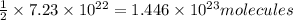

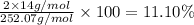
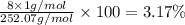

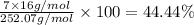
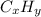
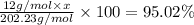
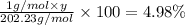
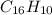 .
.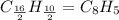 .
.

