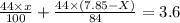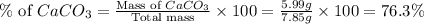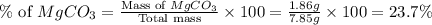Amixture contained calcium carbonate and magnesium carbonate in unspecified proportions. a 7.85g sample of this mixture has reacted with excess hydrochloric acid, producing 1.94l of carbon dioxide at 25 degrees c and 785mmhg. what are the percentage of calcium carbonate and magnesium carbonate in the sample?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1

Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1


Chemistry, 23.06.2019 03:30
27 drag each label to the correct location on the image. a particular exosolar system has five planets in total: a, b, c, d, and e. the table lists the orbital periods of these planets in days. planet orbital period (days) a 600 b 80 c 1,000 d 500 e 100 move each planet to its orbit in the system.
Answers: 3
You know the right answer?
Amixture contained calcium carbonate and magnesium carbonate in unspecified proportions. a 7.85g sam...
Questions

Biology, 02.12.2020 01:30


Mathematics, 02.12.2020 01:30


Mathematics, 02.12.2020 01:30



English, 02.12.2020 01:30


Biology, 02.12.2020 01:30

History, 02.12.2020 01:30



Chemistry, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30

Computers and Technology, 02.12.2020 01:30

Arts, 02.12.2020 01:30


Chemistry, 02.12.2020 01:30

Mathematics, 02.12.2020 01:30

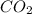 produced ca be calculated from ideal gas equation:
produced ca be calculated from ideal gas equation: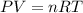
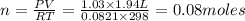
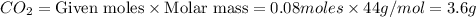
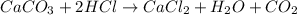
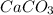 react to give 44 g of
react to give 44 g of 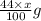 of
of 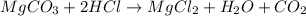
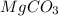 react to give 44 g of
react to give 44 g of 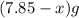 of
of 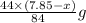 of
of