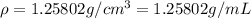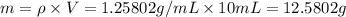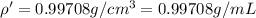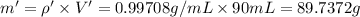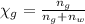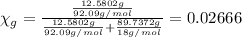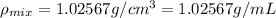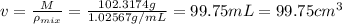You mix 10 ml glycerol and 90 ml water to obtain a 10% glycerol solution. the density of the mixture is ρmix = 1.02567 g/cm. what are the mole fraction of glycerol and the volume of the mixture? what is the reason for the volume change? mm(glycerol) = 92.09 g/mol, mm(h2o) = 18 g/mol, ρ(glycerol) = 1.25802 g/cm3, ρ(h2o) = 0.99708 g/cm3.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 10:10
When electrolyzing copper (ll) chloride, what reaction takes place at the anode? what reaction takes place at the cathode?
Answers: 1

Chemistry, 22.06.2019 15:20
Draw any one of the skeletal structures of a 2° alkyl bromide having the molecular formula of c6h13br and two stereogenic centers. indicate chirality by using wedge and hashed wedge notation. lone pairs do not need to be shown.
Answers: 1

You know the right answer?
You mix 10 ml glycerol and 90 ml water to obtain a 10% glycerol solution. the density of the mixture...
Questions

Mathematics, 25.08.2019 21:00

Health, 25.08.2019 21:00

Health, 25.08.2019 21:00


Social Studies, 25.08.2019 21:00



History, 25.08.2019 21:00


Biology, 25.08.2019 21:00

Business, 25.08.2019 21:00

English, 25.08.2019 21:00


Mathematics, 25.08.2019 21:00


Social Studies, 25.08.2019 21:00




Biology, 25.08.2019 21:00