Chemistry, 05.07.2019 18:20 manlyman31
What volume (in liters) of carbon monoxide gas, measured at a temperature of 212 k and a pressure of 676 mm hg, is required to synthesize 19.3 g of methanol. how many liters of oxygen (at stp) are required to form 12.5 g of h2o ? show your work

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Turbo the snail moves across the ground at a pace of 12 feet per day. if the garden is 48 feet away, how many days will it take for the snail to get there?
Answers: 2

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

Chemistry, 22.06.2019 17:30
What type of organic molecule comprises the majority of a potato?
Answers: 1

You know the right answer?
What volume (in liters) of carbon monoxide gas, measured at a temperature of 212 k and a pressure of...
Questions


History, 18.01.2020 21:31

Mathematics, 18.01.2020 21:31

Physics, 18.01.2020 21:31


Biology, 18.01.2020 21:31

English, 18.01.2020 21:31

English, 18.01.2020 21:31

English, 18.01.2020 21:31


History, 18.01.2020 21:31





Mathematics, 18.01.2020 21:31

History, 18.01.2020 21:31

History, 18.01.2020 21:31

Physics, 18.01.2020 21:31

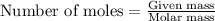 ......(1)
......(1)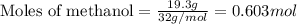
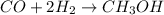
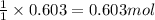 of carbon monoxide.
of carbon monoxide.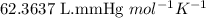
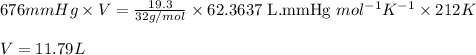
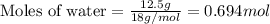
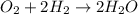
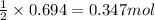 of oxygen gas.
of oxygen gas.