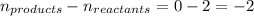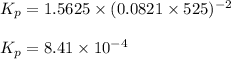Chemistry, 05.07.2019 18:20 aliceohern
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting initially of 1.8 moles of co and 2.2 moles of h2 in 5.0-l container was found to contain 0.6 moles of ch3oh after reaching equilibrium (a) calculate equilibrium concentration (in molarity) of co and h2 (b) calculate equilibrium constants kc and kp for this reaction

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 23.06.2019 00:00
Which is true about metals used for jewelry, such as platinum and gold? a. they have low flammability. b. they have low reactivity. c. they have high flammability. d. they have high reactivity.
Answers: 1

Chemistry, 23.06.2019 04:10
An unknown substance has been shown to have metallic bonds. which of the following is most likely a property of this substance? a. low conductivity b. low boiling point c. high malleability d. high solubility in water
Answers: 2

Chemistry, 23.06.2019 19:00
X-rays are used instead of visible light because x-rays have shorter than visible light, allowing them to produce images of much greater detail. a) energies b) frequencies c) speeds d) wavelengths
Answers: 3
You know the right answer?
Methanol can be synthesized from monoxide and hydrogen gas at 525 k. a reaction mixture consisting i...
Questions




Mathematics, 09.02.2022 19:00




Mathematics, 09.02.2022 19:00






Mathematics, 09.02.2022 19:10



Social Studies, 09.02.2022 19:10


SAT, 09.02.2022 19:10

Mathematics, 09.02.2022 19:10

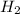 are 0.24 M and 0.32 M.
are 0.24 M and 0.32 M.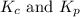 are 1.5625 and
are 1.5625 and 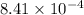
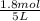
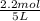
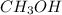 = 0.6
= 0.6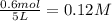
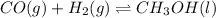
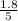
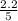 0
0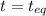
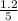
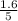
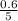
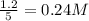
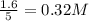
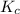 for the given chemical reaction follows:
for the given chemical reaction follows:![K_c=\frac{[CH_3OH]}{[CO][H_2]}](/tpl/images/0054/9747/63b2d.png)
![[CH_3OH]=0.12mol/L](/tpl/images/0054/9747/f965f.png)
![[CO]=0.24mol/L](/tpl/images/0054/9747/ee0b0.png)
![[H_2]=0.32mol/L](/tpl/images/0054/9747/aa037.png)
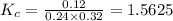
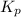 with
with 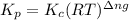
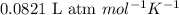
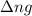 = change in number of moles of gas particles =
= change in number of moles of gas particles =