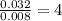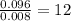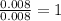Chemistry, 05.07.2019 18:20 hallmansean04
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after complete combustion, a 0.7020 g sample of the compound yields 1.4 g of co2, 0.86 g of h2o, and 0.478 g of sio2. what is the empirical formula of the compound?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 20:00
The volume of a single vanadium atom is 9.29×10-24 cm3. what is the volume of a vanadium atom in microliters?
Answers: 3

Chemistry, 23.06.2019 12:30
What would happen to a weak base dissociation equilibrium if more products we added
Answers: 1
You know the right answer?
Asample containing only carbon, hydrogen, and silicon is subjected to elemental analysis. after comp...
Questions




Computers and Technology, 22.11.2019 05:31








Mathematics, 22.11.2019 05:31







Mathematics, 22.11.2019 05:31

Mathematics, 22.11.2019 05:31

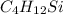 .
.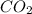 = 1.4 g
= 1.4 g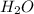 = 0.86 g
= 0.86 g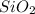 = 0.478 g
= 0.478 g 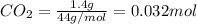
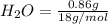 =0.048mol
=0.048mol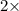 Moles of
Moles of 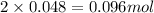
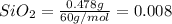 mol
mol