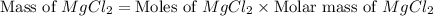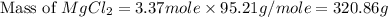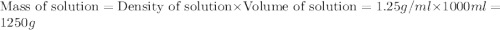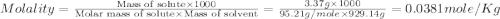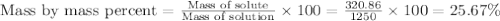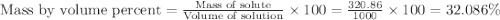Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 12:10
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution.calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1

Chemistry, 22.06.2019 14:40
Pastoral farming is best described as a. a method of raising livestock and moving herds b. an african method of agriculture c. a method of cultivating crops on poor soils d. a common method of desert farming select the best answer from the choices provided a b c d
Answers: 2

Chemistry, 22.06.2019 15:00
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1
You know the right answer?
The density of a 3.37m mgcl2 (fw = 95.21) is 1.25 g/ml. calulate the molality, mass/mass percent, an...
Questions

English, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

English, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

Social Studies, 05.02.2021 06:10


English, 05.02.2021 06:10



Mathematics, 05.02.2021 06:10



Physics, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

Mathematics, 05.02.2021 06:10

English, 05.02.2021 06:10

Business, 05.02.2021 06:10

English, 05.02.2021 06:10

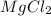 (solute) = 95.21 g/mole
(solute) = 95.21 g/mole