
Chemistry, 05.07.2019 18:30 bikkiecuanas13
Be sure to answer all parts. one gallon of gasoline in an automobiles engine produces on average 9.50 kg of carbon dioxide, which is a greenhouse gas; that is, it promotes the warming of earth's atmosphere. calculate the annual production of carbon dioxide in kilograms if there are exactly 40.0 million cars in the united states and each car covers a distance of 5790 mi at a consumption rate of 24.1 miles per gallon. enter your answer in scientific notation. × 10 kg

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3


Chemistry, 23.06.2019 03:10
Which is true according to the law of conservation of energy
Answers: 1
You know the right answer?
Be sure to answer all parts. one gallon of gasoline in an automobiles engine produces on average 9.5...
Questions

Mathematics, 07.07.2019 05:00



History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00

History, 07.07.2019 05:00



Mathematics, 07.07.2019 05:00

Mathematics, 07.07.2019 05:00


History, 07.07.2019 05:00

Biology, 07.07.2019 05:00

Mathematics, 07.07.2019 05:00





History, 07.07.2019 05:00

 .
.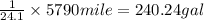
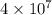
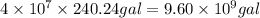
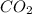
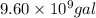 of gasoline will produce:
of gasoline will produce: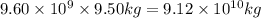 of
of 

