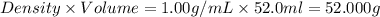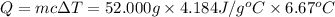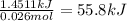Chemistry, 05.07.2019 19:10 angelolucero146
When 26.0 ml of 0.500 m h2so4 is added to 26.0 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50°c, the temperature rises to 30.17°c. calculate δh of this reaction. (assume that the total volume is the sum of the individual volumes and that the density and specific heat capacity of the solution are the same as for pure water.) (d for water = 1.00 g/ml; c for water = 4.184 j/g·°c.)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 22:30
What must be in balance for temperatures to remain constant?
Answers: 1

Chemistry, 23.06.2019 08:00
Problem page a jet airplane reaches 846. km/h on a certain flight. what distance does it cover in 13.0 min? set the math up. but don't do any of it. just leave your answer as a math expression. also, be sure your answer includes all the correct unit symbols.
Answers: 2

Chemistry, 23.06.2019 14:00
Comparing john newland’s octaves with the modern periodic table, which 5 elements have been discovered between hydrogen and iron since newland’s time?
Answers: 3
You know the right answer?
When 26.0 ml of 0.500 m h2so4 is added to 26.0 ml of 1.00 m koh in a coffee-cup calorimeter at 23.50...
Questions







Computers and Technology, 07.11.2019 06:31



Advanced Placement (AP), 07.11.2019 06:31




English, 07.11.2019 06:31





Mathematics, 07.11.2019 06:31

Mathematics, 07.11.2019 06:31

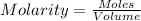
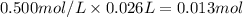
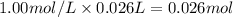
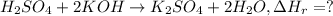
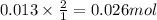 of potassium hydroxide.
of potassium hydroxide.