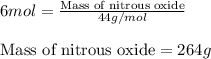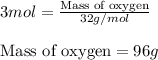Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a) nitrous oxide and (b) air. compare the required number of moles and the oxidizer mass using each of the two oxidizers for the complete, stoichiometric combustion of ethylene.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Me i dont know what to do! the table compares the number of electrons in two unknown neutral atoms. comparison of electrons atom number of electrons a 10 d 11 use this information to determine the number of valence electrons in the atoms. which of the following correctly compares the stability of the two atoms? both are unreactive. both are highly reactive. a is unreactive and d is reactive. a is reactive and d is unreactive.
Answers: 1

Chemistry, 22.06.2019 10:00
Why is the structure of molecule important to its function?
Answers: 1

Chemistry, 22.06.2019 12:30
What is the percent composition of ca(oh)2? 37.7% ca, 53.0% o, and 10.3% h 45.5% ca, 38.2% o, and 16.3% h 54.0% ca, 43.0% o, and 2.7% h 64.7% ca, 27.0% o, and 8.3% h
Answers: 2

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2
You know the right answer?
Write the balanced chemical equation for the complete, stoichiometric combustion of ethylene in (a)...
Questions


Biology, 26.09.2019 12:30


History, 26.09.2019 12:30


Mathematics, 26.09.2019 12:30


Health, 26.09.2019 12:30

History, 26.09.2019 12:30

Mathematics, 26.09.2019 12:30

SAT, 26.09.2019 12:30

Biology, 26.09.2019 12:30




History, 26.09.2019 12:30

Mathematics, 26.09.2019 12:30

History, 26.09.2019 12:30

Chemistry, 26.09.2019 12:30

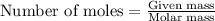 ....(1)
....(1)