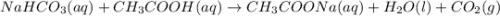Chemistry, 05.07.2019 20:20 jayjinks976
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. the two substances react, releasing carbon dioxide gas to the atmosphere. after the reaction, the contents of the reaction vessel weigh 11.6 g. what is the mass of carbon dioxide released during the reaction?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:40
During which time interval does the object travel approximately 10 meters
Answers: 3

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2


You know the right answer?
A3.4 g sample of sodium hydrogen carbonate is added to a solution of acetic acid weighing 10.9 g. th...
Questions

Mathematics, 16.03.2020 03:04


Mathematics, 16.03.2020 03:04

Chemistry, 16.03.2020 03:05



Physics, 16.03.2020 03:05

Biology, 16.03.2020 03:05

Physics, 16.03.2020 03:05

Mathematics, 16.03.2020 03:05



History, 16.03.2020 03:05

Mathematics, 16.03.2020 03:05



Mathematics, 16.03.2020 03:06

Biology, 16.03.2020 03:06


Social Studies, 16.03.2020 03:06