The equilibrium constant, kc, for the reaction
n2o4(g)? 2no2(g)
is 4.9×10? 3.
if t...

Chemistry, 05.07.2019 20:20 ronaldhernandez598
The equilibrium constant, kc, for the reaction
n2o4(g)? 2no2(g)
is 4.9×10? 3.
if the equilibrium mixture contains [no2] = 0.050 m , what is the molar concentration of n2o4?
express the concentration to two significant figures and include the appropriate units.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 21:40
Tooth enamel consists mainly of the mineral calcium hydroxyapatite, ca_10(po_4)_6(oh)_2. trace elements in teeth of archaeological specimens provide anthropologist with clues about diet and diseases of ancient people. students at hamline university measured strontium in enamel from extracted wisdom teeth by atomic absorption spectroscopy. solutions with a constant total volume of 10.0 ml contained 0.726 mg of dissolved tooth enamel plus variable concentrations of added sr. added sr find the concentration of sr in the 10 ml sample solution in parts per billion = ng/ml. find the concentration of sr in tooth enamel in parts per million = mu g/g.
Answers: 2


Chemistry, 23.06.2019 02:00
Calculate the molarity of each aqueous solution: a. 78.0 ml of 0.240 m naoh diluted to 0.250 l with water b. 38.5 ml of 1.2 m hno3 diluted to 0.130 l with water
Answers: 1

Chemistry, 23.06.2019 04:10
In an experiment, 45g of silicon tetrachloride are treated with 45ml of water. what is the theoretical yield in grams of hcl
Answers: 3
You know the right answer?
Questions


Mathematics, 26.08.2021 20:30



Mathematics, 26.08.2021 20:30






English, 26.08.2021 20:30

Health, 26.08.2021 20:30


Mathematics, 26.08.2021 20:30

English, 26.08.2021 20:30

Geography, 26.08.2021 20:30

Computers and Technology, 26.08.2021 20:30

Chemistry, 26.08.2021 20:30


Mathematics, 26.08.2021 20:30

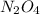 is,
is, 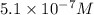

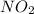 = 0.050 M
= 0.050 M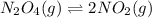
![K_c=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0055/3008/271f5.png)
![4.9\times 10^3=\frac{(0.050)^2}{[N_2O_4]}](/tpl/images/0055/3008/a5c3c.png)
![[N_2O_4]=5.1\times 10^{-7}M](/tpl/images/0055/3008/c9f7d.png)


