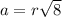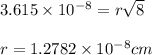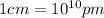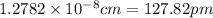Chemistry, 05.07.2019 20:20 bryson9604
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) calculate the mass of one unit cell of copper (in grams) b.) calculate the volume of the copper unit cell (in cm3). c.) calculate the edge length of the unit cell (in cm). d.) calculate the radius of a copper atom (in pm).

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:30
The clouds are grey and ground is wet. a quantitative b qualitative
Answers: 1

Chemistry, 22.06.2019 05:00
Choose all the answers that apply. ionic compounds dissolve easily in water do not dissolve in water have low melting points have high melting points conduct electricity when melted
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1
You know the right answer?
Copper crystallizes with a face-centered cubic lattice and has a density of 8.93 g/cm3.
a.) ca...
a.) ca...
Questions

Mathematics, 09.02.2021 21:20




Mathematics, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20



Biology, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20

Chemistry, 09.02.2021 21:20

Computers and Technology, 09.02.2021 21:20

Mathematics, 09.02.2021 21:20


Mathematics, 09.02.2021 21:20



Mathematics, 09.02.2021 21:20

History, 09.02.2021 21:20

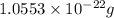
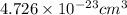
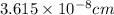
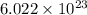 number of atoms.
number of atoms.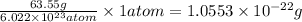
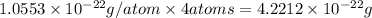
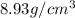
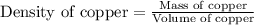
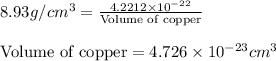
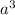
![\sqrt[3]{4.726\times 10^{-23}}cm^3=3.615\times 10^{-8}cm](/tpl/images/0055/3009/53021.png)