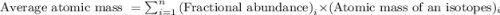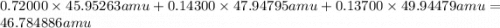Chemistry, 05.07.2019 20:30 SilverTheAmarok
On another planet, the isotopes of titanium have the given natural abundances. isotope abundance mass (amu) 46ti 72.000% 45.95263 48ti 14.300% 47.94795 50ti 13.700% 49.94479 what is the average atomic mass of titanium on that planet?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:50
Express the following number in scientific notation. 0.026890 =
Answers: 1

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2
You know the right answer?
On another planet, the isotopes of titanium have the given natural abundances. isotope abundance mas...
Questions

Physics, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43




Computers and Technology, 27.10.2019 04:43

Biology, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43




Chemistry, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43



Chemistry, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43

Mathematics, 27.10.2019 04:43