
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2 ch2cl2 (g) calculate the equilibrium concentrations of reactants and product when 0.377 moles of ch4 and 0.377 moles of ccl4 are introduced into a 1.00 l vessel at 350 k.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
If you burn 10 kilograms of wood in a fire (combustion) what is the weight of the products after the fire has finished burning the wood?
Answers: 3

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1


Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
The equilibrium constant, kc, for the following reaction is 9.52×10-2 at 350 k. ch4 (g) + ccl4 (g) 2...
Questions

History, 19.07.2019 16:50

Health, 19.07.2019 16:50





History, 19.07.2019 16:50




Social Studies, 19.07.2019 16:50

English, 19.07.2019 16:50

Social Studies, 19.07.2019 16:50




Mathematics, 19.07.2019 16:50

Chemistry, 19.07.2019 16:50

Physics, 19.07.2019 16:50

Physics, 19.07.2019 16:50

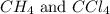 is 0.377 M and equilibrium concentration of
is 0.377 M and equilibrium concentration of 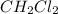 is 0.116 M
is 0.116 M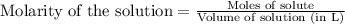
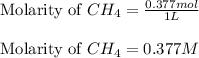

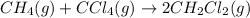
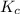 for the given equation follows:
for the given equation follows:![K_c=\frac{[CH_2Cl_2]^2}{[CH_4][CCl_4]}](/tpl/images/0055/3628/bf52a.png)
![K_c=9.52\times 10^{-2}\\[CH_4]=0.377M\\[CCl_4]=0.377M](/tpl/images/0055/3628/8ee11.png)
![9.52\times 10^{-2}=\frac{[CH_2Cl_2]^2}{(0.377)\times (0.377)}](/tpl/images/0055/3628/5aafe.png)
![[CH_2Cl_2]=0.116M](/tpl/images/0055/3628/7ed7d.png)


