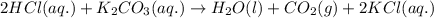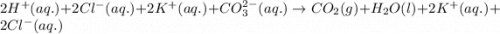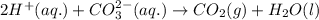Chemistry, 05.07.2019 22:10 chloeholt123
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express your answer as a chemical equation. identify all of the phases in your answer.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:30
Phosphorous acid, h3po3(aq) , is a diprotic oxyacid that is an important compound in industry and agriculture. the values of phosphorous acid are 1.30 6.70 calculate the ph for each of the given points in the titration of 50.0 ml of 1.5 m h3po3(aq) with 1.5 m koh(aq) .
Answers: 3

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

You know the right answer?
Enter the balanced complete ionic equation for hcl(aq)+k2co3(aq)→h2o(l)+co2(g)+kcl (aq). express you...
Questions

Mathematics, 17.09.2019 05:10












Mathematics, 17.09.2019 05:10



Mathematics, 17.09.2019 05:10

History, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Mathematics, 17.09.2019 05:10

Health, 17.09.2019 05:10