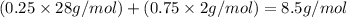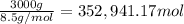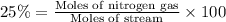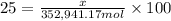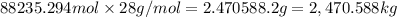Chemistry, 05.07.2019 22:10 tarhondaeiland4122
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fow rate of the stream is 3000 kg/h. calculate the rate of flow of nitrogen into the reactor in kg/h. (suggestion: first calculate the average molecular weight of the mixture.)

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1

Chemistry, 23.06.2019 03:00
You have a sample of a metal, the sample is exactly 6.02 x 1023atom, if the sample has a mass 55.85 what metal is your sample made of?
Answers: 2

Chemistry, 23.06.2019 10:30
Silver is a white metal that is an excellent conductor. silver tarnishes when exposed to air and light. the density of silver is 10.49 g/cm3. the melting point is 962oc and the boiling point is 2000oc. a chemical property of silver is
Answers: 3
You know the right answer?
The feed to an ammonia synthesis reactor contains 25 mole% nitrogen and the balance hydrogen. the fo...
Questions





English, 23.06.2019 10:10


English, 23.06.2019 10:10

Health, 23.06.2019 10:10


English, 23.06.2019 10:10

Mathematics, 23.06.2019 10:10


Mathematics, 23.06.2019 10:10


Mathematics, 23.06.2019 10:10

Mathematics, 23.06.2019 10:10