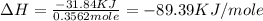Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was neutralized by 137 cm3 of 2.6 mol dm-3 naoh. the temperature rose from 298 k to 325.8 k. the specific heat capacity is the same as water, 4.18 j/k g.
a. 44.69 kj/mol
b. 6123.06 kj/mol
c. 597.46 kj/mol
d. 89.39 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
This drawing shows a human body system. what is the primary function of this body system?
Answers: 3

Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1

Chemistry, 22.06.2019 23:50
Which scientists contributed to the determination of how cfcs in clouds in the upper atmosphere could destroy ozone molecules
Answers: 1
You know the right answer?
Find the enthalpy of neutralization of hcl and naoh. 137 cm3 of 2.6 mol dm-3 hydrochloric acid was n...
Questions



Advanced Placement (AP), 28.02.2020 20:23

Mathematics, 28.02.2020 20:23


Mathematics, 28.02.2020 20:23


Spanish, 28.02.2020 20:23




Geography, 28.02.2020 20:23

Mathematics, 28.02.2020 20:23


Mathematics, 28.02.2020 20:23


Law, 28.02.2020 20:23



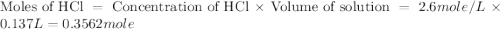
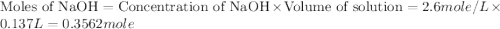

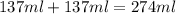
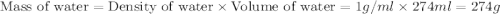
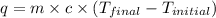
 = specific heat of water =
= specific heat of water = 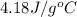
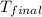 = final temperature of water = 325.8 K
= final temperature of water = 325.8 K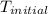 = initial temperature of metal = 298 K
= initial temperature of metal = 298 K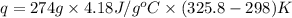
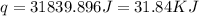
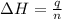
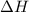 = enthalpy of neutralization = ?
= enthalpy of neutralization = ?