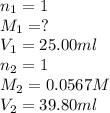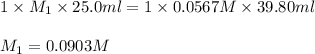Chemistry, 06.07.2019 00:20 cocothunder635
Formic acid (hco2h, ka = 1.8 × 10-4) is the principal component in the venom of stinging ants. what is the molarity of a formic acid solution if 25.00 ml of the formic acid solution requires 39.80 ml of 0.0567 m naoh to reach the equivalence point?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 11:00
3) in peaches, [oh]=3.16x10-11 m a) find [h+ ] b) what is the ph? c) is the solution acidic, basic, or neutral?
Answers: 1


Chemistry, 22.06.2019 13:30
What produces wave a)sound b) heats c)transfer of energy d)vibrations
Answers: 2
You know the right answer?
Formic acid (hco2h, ka = 1.8 × 10-4) is the principal component in the venom of stinging ants. what...
Questions



World Languages, 27.01.2020 18:31

Spanish, 27.01.2020 18:31

Health, 27.01.2020 18:31

Mathematics, 27.01.2020 18:31


Biology, 27.01.2020 18:31




Mathematics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31

Physics, 27.01.2020 18:31


Mathematics, 27.01.2020 18:31


Biology, 27.01.2020 18:31

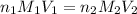
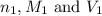 are the n-factor, molarity and volume of formic acid which is
are the n-factor, molarity and volume of formic acid which is 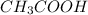
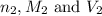 are the n-factor, molarity and volume of sodium hydroxide base which is NaOH.
are the n-factor, molarity and volume of sodium hydroxide base which is NaOH.