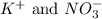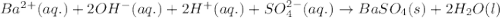Chemistry, 06.07.2019 00:30 danielle413
When the following two solutions are mixed:
k2co3(aq)+fe(no3)3(aq)
the mixture contains the ions listed below. sort these species into spectator ions and ions that react.
drag the appropriate items to their respective bins.
no3-)aq), fe3+ , co3 2-, k+
part b
what is the correct net ionic equation, including all coefficients, charges, and phases, for the following set of reactants? assume that the contribution of protons from h2so4 is near 100 %.
ba(oh)2(aq)+h2so4(aq)?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:10
When water dissociates, each water molecule splits into a hydroxide ion and a) h 3 o + b) a hydrogen atom c) a hydrogen ion d) h 2 o e) oh —
Answers: 2


Chemistry, 22.06.2019 12:00
Explain what happens at the saturation point when adding salt to water at room temperature.
Answers: 1

Chemistry, 22.06.2019 13:00
12. calculate the hydroxide ion concentration of a solution with ph = 3.25. show all calculations leading to an answer
Answers: 3
You know the right answer?
When the following two solutions are mixed:
k2co3(aq)+fe(no3)3(aq)
the mixture contains...
k2co3(aq)+fe(no3)3(aq)
the mixture contains...
Questions

Mathematics, 18.03.2021 01:00

Biology, 18.03.2021 01:00


Health, 18.03.2021 01:00

English, 18.03.2021 01:00



Mathematics, 18.03.2021 01:00




Mathematics, 18.03.2021 01:00






History, 18.03.2021 01:00

History, 18.03.2021 01:00

Chemistry, 18.03.2021 01:00

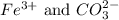 and the ions that react are
and the ions that react are