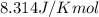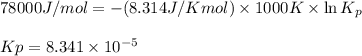Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Which element would mostly likely have an electron affinity measuring closest to zero
Answers: 3

Chemistry, 22.06.2019 12:00
Give the set of reactants (including an alkyl halide and a nucleophile) that could be used to synthesize the following ether: draw the molecules on the canvas by choosing buttons from the tools (for bonds and charges), atoms, and templates toolbars, including charges where needed. ch3ch2och2ch2chch3 | ch3
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 04:40
[01.07]what is the answer to the problem: 101 g + 25.01 g + 5.05 g? 131.06 g 131.1 g 131 g 130 g
Answers: 1
You know the right answer?
The free energy of formation of nitric oxide, no, at 1000 k (roughly the temperature in an automobil...
Questions




Mathematics, 21.04.2020 21:42

Biology, 21.04.2020 21:42



German, 21.04.2020 21:42

Mathematics, 21.04.2020 21:42

Mathematics, 21.04.2020 21:42






History, 21.04.2020 21:43

Mathematics, 21.04.2020 21:43


Social Studies, 21.04.2020 21:43

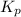 for the chemical equation is
for the chemical equation is 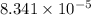
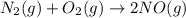
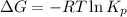
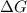 = Gibbs free energy = 78 kJ/mol = 78000 J/mol (Conversion factor: 1kJ = 1000J)
= Gibbs free energy = 78 kJ/mol = 78000 J/mol (Conversion factor: 1kJ = 1000J)