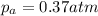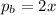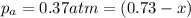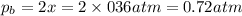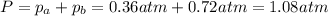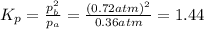Chemistry, 06.07.2019 02:20 wannaoneisforever
Consider the hypothetical reaction a((g). a flask is charged with 0.73atm of pure a, after which it is allowed to reach equilibrium at 0 ? c. at equilibrium the partial pressure of a is 0.37atm .
a: what is the total pressure in the flask at equilibrium?
b: what is the value of kp?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which of these properties, used alone, would be least useful in identifying most minerals? a. color b. luster c. streak d. density
Answers: 2

Chemistry, 22.06.2019 07:30
Calculate the ratio of h+ ions to oh– ions at a ph = 7. find the concentration of h+ ions to oh– ions listed in table b of your student guide. then divide the h+ concentration by the oh– concentration. record this calculated ratio in table a of your student guide. compare your approximated and calculated ratios of h+ ions to oh– ions at a ph = 7. are they the same? why or why not? record your comparison in table a. what is the concentration of h+ ions at a ph = 7? mol/l what is the concentration of oh– ions at a ph = 7? mol/l what is the ratio of h+ ions to oh– ions at a ph = 7? : 1
Answers: 1

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 15:20
Which description best characterizes the motion of particles in a solid?
Answers: 2
You know the right answer?
Consider the hypothetical reaction a((g). a flask is charged with 0.73atm of pure a, after which it...
Questions





Social Studies, 27.01.2020 22:31



History, 27.01.2020 22:31

Computers and Technology, 27.01.2020 22:31

Computers and Technology, 27.01.2020 22:31


Computers and Technology, 27.01.2020 22:31





Computers and Technology, 27.01.2020 22:31



 .
.