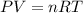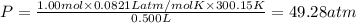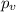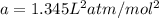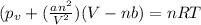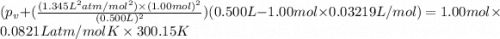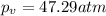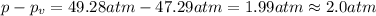Chemistry, 06.07.2019 05:10 ramberson101
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference between the ideal pressure (as predicted by the ideal gas law) and the real pressure (as predicted by the van der waals equation)? for argon, a=1.345(l2⋅atm)/mol2 and b=0.03219l/mol. express your answer to two significant figures and include the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 05:40
Consider the elements bromine and chlorine; which elements has a larger ionic radius ?
Answers: 1

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 01:30
Polar bears give birth and hunt on sea ice. which of the following would polar bears survive during the melting of arctic ice? growing another layer of fur during summer migrate inland to search for different food sources staying put until the ice refreezes sticking to the usual diet of seals
Answers: 1
You know the right answer?
If 1.00 mol of argon is placed in a 0.500-l container at 27.0 degree c , what is the difference betw...
Questions


Arts, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00


History, 03.12.2020 01:00

Chemistry, 03.12.2020 01:00

Geography, 03.12.2020 01:00



Mathematics, 03.12.2020 01:00







Mathematics, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00

Chemistry, 03.12.2020 01:00

Mathematics, 03.12.2020 01:00