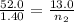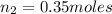Chemistry, 06.07.2019 19:10 imeldachavez124
Asample of gas in a cylinder as in the example in part a has an initial volume of 52.0 l , and you have determined that it contains 1.40 moles of gas. the next day you notice that some of the gas has leaked out. the pressure and temperature remain the same, but the volume has changed to 13.0 l . how many moles of gas (n2) remain in the cylinder?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Given the following reaction: c3h8+5o2=3co2+4h20 how many grams of co2 will be produced 7 g of c3h8 and 98 g of o2
Answers: 1


Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 18:30
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2
You know the right answer?
Asample of gas in a cylinder as in the example in part a has an initial volume of 52.0 l , and you h...
Questions







Mathematics, 25.07.2019 00:00


Chemistry, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00

Chemistry, 25.07.2019 00:00

Mathematics, 25.07.2019 00:00




Health, 25.07.2019 00:00




Mathematics, 25.07.2019 00:00

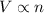 (At constant temperature and pressure)
(At constant temperature and pressure) 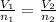
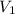 = initial volume of gas = 52.0 L
= initial volume of gas = 52.0 L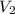 = final volume of gas = 13.0 L
= final volume of gas = 13.0 L
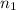 = initial number of moles = 1.40
= initial number of moles = 1.40 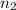 = final number of moles = ?
= final number of moles = ?