
Chemistry, 06.07.2019 21:20 mikailah0988
How many moles of al2o3 can be produced from the reaction of 10.0 g of al and 19.0 of o2?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:20
Which of the following is true for the actual yield of a reaction? it is always calculated as a ratio. it is the yield from the excess reactant. it is the yield from the limiting reactant. it is always less than the theoretical yield.
Answers: 1

Chemistry, 22.06.2019 17:30
In a heat of an engine, if 700 j enters the system, and the piston does 400 j of work what is the final internal (thermal) energy of the system if the initial energy is 1,500 j
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3

Chemistry, 22.06.2019 22:00
Pls ill give u brainliest which of the following is true about science? 1. political conditions are unable to influence it. 2. economic concerns may prevent it from solving problems.
Answers: 2
You know the right answer?
How many moles of al2o3 can be produced from the reaction of 10.0 g of al and 19.0 of o2?...
Questions

Mathematics, 01.08.2019 23:30





History, 01.08.2019 23:30


Physics, 01.08.2019 23:30



Mathematics, 01.08.2019 23:30









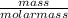
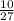
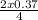 = 0.185moles of Al₂O₃
= 0.185moles of Al₂O₃


