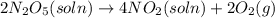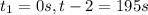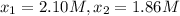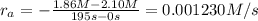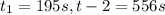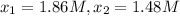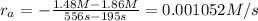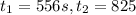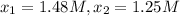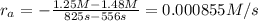The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2...

Chemistry, 07.07.2019 03:10 natalie2sheffield
The decomposition of n2o5 can be described by the equation.
2n2o5 (soln) > 4no2 (soln) + 2 (g)
given this data for the reaction at 45 degrees c in carbon tetrachloride solution, calculate the average rate for each successive time interval.
t(s) [n2o5] (m)
0 2.10
195 1.86
556 1.48
825 1.25
interval: 0 s to 195 s
reaction rate= /s
195 s to 556 s
reaction rate= /s
556 s to 825 s
reaction rate= /s

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 15:30
Which suspect most likely committed the robbery and how do you know
Answers: 2

Chemistry, 22.06.2019 16:30
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

You know the right answer?
Questions


Mathematics, 21.08.2021 18:40

Mathematics, 21.08.2021 18:40

World Languages, 21.08.2021 18:40

Mathematics, 21.08.2021 18:40

Mathematics, 21.08.2021 18:40



Chemistry, 21.08.2021 18:40

Social Studies, 21.08.2021 18:40


Social Studies, 21.08.2021 18:40

Social Studies, 21.08.2021 18:40


English, 21.08.2021 18:40

Mathematics, 21.08.2021 18:40

English, 21.08.2021 18:40


Mathematics, 21.08.2021 18:50

English, 21.08.2021 18:50

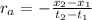
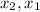 : concentration at time
: concentration at time 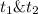 respectively.
respectively.