
Which of the following would increase the rate of a chemical reaction between hydrochloric acid (hcl) and solid zinc metal (zn)?
a. decreasing the concentration of hcl
b. pulverizing the zinc metal into a fine powder
c. performing the reaction at a lower temperature
d. decreasing the amount of zn

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:30
Which is true of the reactants in this displacement reaction? fe + 2hcl fecl2 + h2 a. the reactants are located to the left of the arrow in the chemical equation. b. the reactants contain 1 iron atom, 2 hydrogen atoms, and 1 chlorine atom. c. the reactants are the atoms, molecules, or compounds formed in the reaction. d. the reactants have the same physical and chemical properties as the products.
Answers: 1

Chemistry, 22.06.2019 08:30
If i initially have a gas at a pressure of 12 atm, a volume of 23 liters, and a temperature of 200 k, and then i raise the pressure to 14 atm and increase the temperature to 300 k, what is the new volume of the gas?
Answers: 1

Chemistry, 22.06.2019 12:00
From the options provided for each element below, choose the properties that it may have based on its location in the periodic table fluorine (f): highly reactive nonmetal shiny a conductor
Answers: 1

Chemistry, 22.06.2019 15:20
An alloy contains 66 g of pure zinc. what is the percentage of zinc in the alloy? express your answer to two significant figures and include the appropriate units.
Answers: 1
You know the right answer?
Which of the following would increase the rate of a chemical reaction between hydrochloric acid (hcl...
Questions

Mathematics, 22.04.2020 22:14










Mathematics, 22.04.2020 22:15


Computers and Technology, 22.04.2020 22:15

Mathematics, 22.04.2020 22:15

Mathematics, 22.04.2020 22:15

Biology, 22.04.2020 22:15




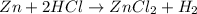
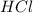 and
and 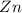 : Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.
: Thus rate of the reaction would decrease on decreasing the concentration of hydrochloric acid and zinc.

