
Chemistry, 08.07.2019 17:30 meramera50
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domestic cooking and heating. (a) balance the following equation representing the combustion of propane in air. include states of matter in your answer. c3h8(g) + o2(g) → co2(g) + h2o(g) (b) how many grams of carbon dioxide can be produced by burning 8.11 moles of propane? assume that oxygen is the excess reactant in this reaction. × 10 g enter your answer in scientific notation.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 02:10
What approach is required to balance the objectives of sustainable development? balancing the objectives of sustainable development requires a(n) .
Answers: 3

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 23.06.2019 00:00
#20 which type of bond is formed when bases pair in dna? ionic bond covalent bond coordinate bond hydrogen bond
Answers: 1
You know the right answer?
Be sure to answer all parts. propane (c3h8) is a minor component of natural gas and is used in domes...
Questions


Mathematics, 14.03.2022 14:00




Mathematics, 14.03.2022 14:00

Health, 14.03.2022 14:00



English, 14.03.2022 14:00





Chemistry, 14.03.2022 14:00




History, 14.03.2022 14:00

Mathematics, 14.03.2022 14:00

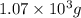
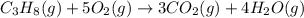
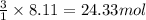 of carbon dioxide gas.
of carbon dioxide gas.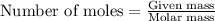
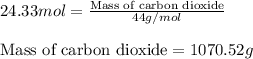
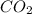 produced in the given reaction and expressed in scientific notation is
produced in the given reaction and expressed in scientific notation is 

