Chemistry, 05.02.2020 11:50 abdullaketbi71
The depletion of ozone (o3) in the stratosphere has been a matter of great concern among scientists in recent years. it is believed that ozone can react with nitric oxide (no) that is discharged from the high-altitude jet plane, the sst. the reaction is
o3 + no > o2 + no2
if 0.827 g of o3 reacts with 0.635 g of no, how many grams of no2 will be produced? g no2 which compound is the limiting reagent? ozone (o3) nitric oxide (no) calculate the number of moles of the excess reagent remaining at the end of the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:00
What is the theoretical yield of carbon dioxide? a)0.993 gb)2.98 gc)3.65 gd)8.93 g
Answers: 1

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1


Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3
You know the right answer?
The depletion of ozone (o3) in the stratosphere has been a matter of great concern among scientists...
Questions







History, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40


Arts, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40





Mathematics, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

Chemistry, 13.11.2020 19:40

Mathematics, 13.11.2020 19:40

 ....(1)
....(1)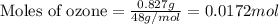
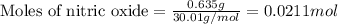
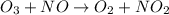
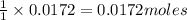 of nitric oxide
of nitric oxide