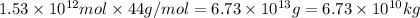Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control. it is prepared by the thermal decomposition of calcium carbonate: caco3(s) → cao(s) co2(g) calculate the yearly release of co2 (in kg) to the atmosphere if the annual production of cao in the united states is 8.6 × 1010 kg.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Calculate the molar mass of aluminum oxide (al2o3). express your answer to four significant figures.
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1


Chemistry, 22.06.2019 19:00
Which statement best describes what happens when molecular compounds melt
Answers: 1
You know the right answer?
Calcium oxide or quicklime (cao) is used in steelmaking, cement manufacture, and pollution control....
Questions




History, 11.04.2020 22:52


History, 11.04.2020 22:52


English, 11.04.2020 22:52

English, 11.04.2020 22:52

Mathematics, 11.04.2020 22:52

Mathematics, 11.04.2020 22:52


Mathematics, 11.04.2020 22:52

Mathematics, 11.04.2020 22:52






Mathematics, 11.04.2020 22:52

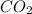 into the atmosphere is
into the atmosphere is 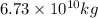 .
.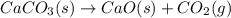
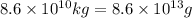
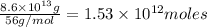
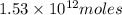 of CaO moles of carbon-dioxide moles produced will be:
of CaO moles of carbon-dioxide moles produced will be: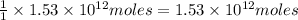 of carbon-dioxide
of carbon-dioxide moles of carbon-dioxide:
moles of carbon-dioxide: