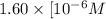Chemistry, 08.07.2019 18:10 PersonPerson13260
Calculate the concentration of h3o⁺ in a solution that contains 6.25 × 10-9 m oh⁻ at 25°c. identify the solution as acidic, basic, or neutral. a) 6.38 × 10-9 m, basic b) 1.60 × 10-6 m, acidic c) 7.94 × 10-11 m, acidic d) 7.38 × 10-3 m, basic e) 4.92× 10-5 m, acidic

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:50
Consider the equilibrium system: 2icl(s) ⇄ i2(s) + cl2(g) which of the following changes will increase the total amount of of cl2 that can be produced? all of the listed answers are correct decreasing the volume of the container removing the cl2 as it is formed adding more icl(s) removing some of the i2(s)
Answers: 1


Chemistry, 22.06.2019 20:00
Acm ruler with main graduations from 1 to 10 from left to right there are 10 secondary graduations between each of the main graduations there is a line that begins. at the left end of the ruler 10 secondary graduations to the left of the “1 main graduation the right end of the line ends on the eighth secondary graduation to the right of 3 how long is the line
Answers: 1

Chemistry, 23.06.2019 02:30
Apound is approximately 0.45 kilogram. a persons weighs 87 kilograms. what is the persons’s weight, in pounds, when expressed to the correct number of significant figures
Answers: 1
You know the right answer?
Calculate the concentration of h3o⁺ in a solution that contains 6.25 × 10-9 m oh⁻ at 25°c. identify...
Questions

Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50


Mathematics, 17.12.2020 22:50


Biology, 17.12.2020 22:50




Advanced Placement (AP), 17.12.2020 22:50


Mathematics, 17.12.2020 22:50


English, 17.12.2020 22:50




Mathematics, 17.12.2020 22:50

Mathematics, 17.12.2020 22:50

Biology, 17.12.2020 22:50

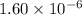 M, acidic
M, acidic![pH=-\log [H_3O^+]](/tpl/images/0066/4841/841e8.png)
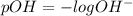
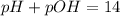
![[OH^-]=6.25\times 10^{-9}M](/tpl/images/0066/4841/a8cb2.png)
![pOH=-log[6.25\times 10^{-9}M]](/tpl/images/0066/4841/b2499.png)
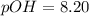
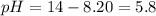
![5.8=-log[H_3O^+]](/tpl/images/0066/4841/59813.png)
![[H_3O^+]=1.60\times [10^{-6}M](/tpl/images/0066/4841/f3b28.png)
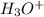 is
is