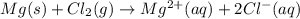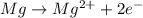Chemistry, 08.07.2019 18:30 mattsucre1823
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent in the redox reaction. mg(s)+cl2(g)⟶mg2+(aq)+2cl−(aq) which substance gets oxidized? mg2+ cl− mg cl2 which substance gets reduced? cl2 mg cl− mg2+ what is the oxidizing agent? cl− mg2+ cl2 mg what is the reducing agent? mg2+ cl2 mg cl−

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 08:30
Identify one disadvantage to each of the following models of electron configuration: -dot structures -arrow and line diagrams -written electron configurations type in your answer below. (answer) -dot structures do not show the distribution of electrons in orbitals and take up a lot of space. -arrow and line diagrams take up a lot of space and make it difficult to count electrons. -written configurations make it easy to lose count of electrons and do not show the distribution of electrons in orbitals.
Answers: 3

Chemistry, 22.06.2019 20:30
Calculate the percent composition by mass of each element in al(oh)3. use at least three significant figures.
Answers: 1

Chemistry, 23.06.2019 03:00
Air pressure is measured in pascals. for a professional american football game, the ball should be inflated to about 90,000 pascals. scientists studied the effects of air temperature on the pressure inside american footballs by taking these steps: 1. prepare 100 footballs. 2. measure each football's air pressure. 3. divide footballs into 10 groups. 4. place the groups in different lockers cooled to different air temperatures. 5. after 12 hours, remove the footballs from lockers. 6. measure each football's pressure again. 7. compare the new pressures to the starting pressures. what two terms best describe the variable "air pressure inside the football" in this experiment? independent, qualitative independent, quantitative dependent, qualitative dependent, quantitative
Answers: 3
You know the right answer?
Identify the oxidized substance, the reduced substance, the oxidizing agent, and the reducing agent...
Questions


History, 12.12.2020 16:50



Chemistry, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

English, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50

Chemistry, 12.12.2020 16:50



Mathematics, 12.12.2020 16:50


English, 12.12.2020 16:50


Mathematics, 12.12.2020 16:50