
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: 2no(g) + o2(g) → 2no2(g)
in one experiment, 0.857 mol of no is mixed with 0.498 mol of o2.
determine which of the two reactants is the limiting reactant. calculate also the number of moles of no2 produced. limiting reactant: moles of no2 produced: moles

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Which statement best describes the he properties of iconic compounds ?
Answers: 1


Chemistry, 23.06.2019 01:00
Imagine if during the cathode ray experiment, the size of the particles of the ray was the same as the size of the atom forming the cathode. which other model or scientific observation would have also been supported? 1. this would support dalton's postulates that proposed the atoms are indivisible because no small particles are involved. 2. this would support bohr's prediction about electrons moving in orbits having specific energy. 3. this would support bohr's prediction about electrons being randomly scattered around the nucleus in the atom. 4. this would support dalton's postulates that proposed that atoms combine in fixed whole number ratios to form compounds.
Answers: 1

Chemistry, 23.06.2019 04:31
Use the drop-down menus to label each of the following changes p for physical change and c for chemical change. the substance changes to a new substance. the original substance can be recovered. the color changes. gas is produced and given off. the substance changes size, shape, or volume.
Answers: 2
You know the right answer?
Be sure to answer all parts. nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2)...
Questions



Health, 12.12.2020 16:40

Health, 12.12.2020 16:40

Health, 12.12.2020 16:40


World Languages, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

History, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40

Biology, 12.12.2020 16:40

Mathematics, 12.12.2020 16:40


Mathematics, 12.12.2020 16:40


English, 12.12.2020 16:40

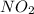 will be produced.
will be produced.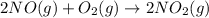
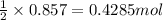 of
of 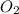
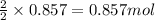 of
of 

