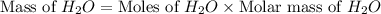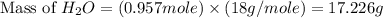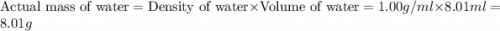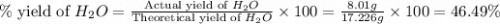Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
City a and city b had two different temperatures on a particular day. on that day, four times the temperature of city a was 8â° c more than 3 times the temperature of city b. the temperature of city a minus twice the temperature of city b was â’3â° c. what was the temperature of city a and city b on that day? city a was 5â° c, and city b was 4â° c. city a was 3â° c, and city b was â’1â° c. city a was 8â° c, and city b was â’3â° c. city a was 5â° c, and city b was â’5â° c.
Answers: 2


Chemistry, 22.06.2019 06:40
Which statement correctly describes metallic bonds? a. they form when certain atoms lose electrons and other atoms gain electrons. b. they involve an attraction between anions and cations. they always involvpoth a metal and a nonmetal. d. they can only form between atoms of the same element. e. they form because electrons can move freely between atoms.
Answers: 3

Chemistry, 22.06.2019 17:30
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
You know the right answer?
What is the percent yield of a reaction in which 74.1 g of tungsten(vi) oxide (wo3) reacts with exce...
Questions



Spanish, 13.11.2019 16:31



English, 13.11.2019 16:31

Mathematics, 13.11.2019 16:31

Mathematics, 13.11.2019 16:31

English, 13.11.2019 16:31


Mathematics, 13.11.2019 16:31

Arts, 13.11.2019 16:31

History, 13.11.2019 16:31


English, 13.11.2019 16:31


Biology, 13.11.2019 16:31



Mathematics, 13.11.2019 16:31

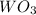 = 74.1 g
= 74.1 g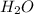 = 18 g/mole
= 18 g/mole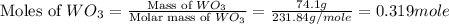
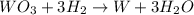
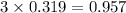 moles of
moles of