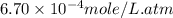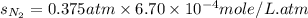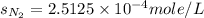Chemistry, 09.07.2019 01:30 glydelxc2780
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical value at high altitude). atmospheric gas mole fraction kh mol/(l*atm) n2 7.81 x 10-1 6.70 x 10-4 o2 2.10 x 10-1 1.30 x 10-3 ar 9.34 x 10-3 1.40 x 10-3 co2 3.33 x 10-4 3.50 x 10-2 ch4 2.00 x 10-6 1.40 x 10-3 h2 5.00 x 10-7 7.80 x 10-4

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Select the correct answer. given: 2libr + ba → babr2 + 2li in this chemical reaction, 325 grams of barium (ba) react completely. how many moles of lithium (li) are produced? a. 1.18 mol b. 2.37 mol c. 4.73 mol d. 16.4 mol e. 32.9 mol
Answers: 2

Chemistry, 22.06.2019 04:20
Which formula can be used to calculate the molar mass of ammonia (nh3)? molar mass of n + molar mass of h 3 × molar mass of n + molar mass of h molar mass of n + 3 × molar mass of h 3 × molar mass of n + 3 × molar mass of h
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30
How might this scientific phenomena be explained? a paper clip floats on water.
Answers: 1
You know the right answer?
Calculate the solubility of nitrogen in water at an atmospheric pressure of 0.480 atm (a typical val...
Questions

Health, 12.02.2020 20:23

Mathematics, 12.02.2020 20:24


Mathematics, 12.02.2020 20:24




History, 12.02.2020 20:26



Mathematics, 12.02.2020 20:29

Social Studies, 12.02.2020 20:30






Mathematics, 12.02.2020 20:30

Chemistry, 12.02.2020 20:30

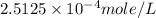
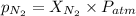
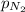 = partial pressure of nitrogen = ?
= partial pressure of nitrogen = ?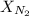 = mole fraction of nitrogen =
= mole fraction of nitrogen = 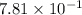
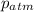 = atmospheric pressure = 0.480 atm
= atmospheric pressure = 0.480 atm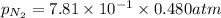
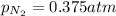
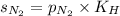
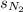 = solubility of nitrogen in water = ?
= solubility of nitrogen in water = ?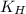 = Henry's constant =
= Henry's constant =