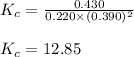Chemistry, 09.07.2019 04:30 dakotalynnwillis01
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.650 m , and [c] = 0.300 m . the following reaction occurs and equilibrium is established: a+2b⇌c at equilibrium, [a] = 0.220 m and [c] = 0.430 m . calculate the value of the equilibrium constant, kc.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Mrs. smith ordered a root beer float (vanilla ice cream + root beer). mrs. smith noticed that the three states of matter (solid, liquid, and gas) all existed simultaneously in her root beer float. a. identify each phase of matter in the root beer float. b. describe the particles of all three phases of matter in the root beer float. (how are the particles arranged and moving? ) c. identify one phase change you would see in a root beer float and described what causes this change.
Answers: 2

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 05:50
Calculate the number of molecules present in 0.750 mol of mgo.
Answers: 3

You know the right answer?
Amixture initially contains a, b, and c in the following concentrations: [a] = 0.350 m , [b] = 0.65...
Questions

English, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00


History, 05.05.2021 01:00

Medicine, 05.05.2021 01:00








Mathematics, 05.05.2021 01:00


English, 05.05.2021 01:00

English, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

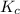 for the given reaction is 12.85.
for the given reaction is 12.85.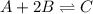
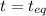 (0.350 - x) (0.650 - 2x) (0.300 + x)
(0.350 - x) (0.650 - 2x) (0.300 + x)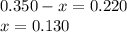
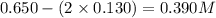
![K_c=\frac{[C]}{[A][B]^2}](/tpl/images/0068/1022/240ef.png)