
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitrogen. 3h2(g) + n2(g) → 2nh3(g) in a particular reaction, 8.00 moles of nh3 were produced. how many moles of h2 and how many moles of n2 were reacted to produce this amount of nh3?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 17:40
Areaction in which products can react to re-form reactants is
Answers: 1

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3

Chemistry, 23.06.2019 00:00
The empirical formula of a compound is ch2o and its mass is 120 amu/molecule, what is its formula?
Answers: 1
You know the right answer?
Ammonia is a principal nitrogen fertilizer. it is prepared by the reaction between hydrogen and nitr...
Questions

Mathematics, 24.02.2021 14:00



Health, 24.02.2021 14:00


Mathematics, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00

Chemistry, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


Chemistry, 24.02.2021 14:00



Mathematics, 24.02.2021 14:00


Mathematics, 24.02.2021 14:00

Mathematics, 24.02.2021 14:00


Chemistry, 24.02.2021 14:00

English, 24.02.2021 14:00

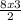 = 12moles
= 12moles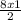 = 4moles of Nitrogen gas
= 4moles of Nitrogen gas

