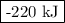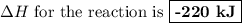Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Un cierto gas tiene un volumen de 800ml a 80°c y 600ml a 80°c y 600mmhg de presión. ¿cual será el volumen del gas a condiciones normales? sí el gas es oxígeno, ¿cuál será su peso? y ¿cuántas moléculas están presentes en el sistema?
Answers: 2

Chemistry, 21.06.2019 23:50
How will the emission of an alpha particle affect the atomic number of an atom
Answers: 3

Chemistry, 22.06.2019 03:00
Schrodinger and heisenberg developed an alternate theory about atomic nature that contradicted some of bohr's model of the atom. how do changes resulting from new technology and evidence affect the reputation of the atomic theory?
Answers: 1

Chemistry, 22.06.2019 11:00
Imagine that twenty i.u.’s of enzyme z were catalyzing the above reaction for one minute, under vmaxconditions, in a 3.00 ml assay volume. the assay is buffered with 20 mm phosphate buffer, ph 7.60. what will the ph be at the end of that one minute?
Answers: 2
You know the right answer?
Given the following data:
c(s) + o2(g) + co2(8) ah = -393 kj
2co(g) + o2(g) → 2c02(8) a...
c(s) + o2(g) + co2(8) ah = -393 kj
2co(g) + o2(g) → 2c02(8) a...
Questions

English, 07.11.2020 02:00

Biology, 07.11.2020 02:00

History, 07.11.2020 02:00


Mathematics, 07.11.2020 02:00





Physics, 07.11.2020 02:00



Biology, 07.11.2020 02:00


Mathematics, 07.11.2020 02:00



Arts, 07.11.2020 02:00