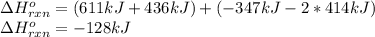Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Write two balanced equations 1. dissolving of solid sodium hydroxide in water 2. the reaction of sodium hydroxide solution with hydrochloric acid
Answers: 1

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 04:00
Gymnast always perform on padded mats. how does the mats protect the gymnast
Answers: 2

Chemistry, 22.06.2019 05:50
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2
You know the right answer?
Use the bond energies provided to estimate δh°rxn for the reaction below. c2h4(g) + h2(g) → c2h6(g)...
Questions


Chemistry, 20.11.2020 23:30

Mathematics, 20.11.2020 23:30




Chemistry, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30


English, 20.11.2020 23:30


Mathematics, 20.11.2020 23:30

English, 20.11.2020 23:30

Mathematics, 20.11.2020 23:30



Physics, 20.11.2020 23:30


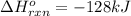
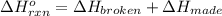
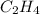 a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain:
a double bond between carbons is broken as well as a bond between hydrogens (such values turn out positive). Furthermore, a single bond between carbons and two single bonds between carbon and hydrogen are made (such values turn out negative), in such a way, we develop the aforesaid equation to obtain: