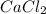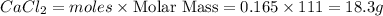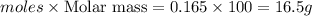When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water are produced.
caco3 + 2hcl ⟶cacl2 + h2o + co2
a) how many grams of calcium chloride will be produced when 26.0g of calcium carbonate are combined whith 12.0g of hydrochloric acid?
b) which reactant is in excess and how many grams of this reactant will remain after the reaction is complete?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 09:30
One way that radioactive waste is treated is by burying it in repositories. the repositories are found only in states with very low populations. true or false? a. trueb. false(also i meant to put high school but it put down middle school instead)
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
When calcium carbonate is added to hydrochloric acid, calcium chloride, carbon dioxide, and water ar...
Questions








History, 02.11.2020 16:30



History, 02.11.2020 16:30







Mathematics, 02.11.2020 16:30


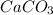 is the excess reagent and 16.5g of
is the excess reagent and 16.5g of 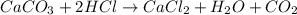
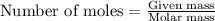
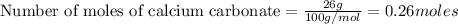
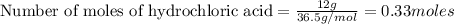
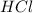 react with 1 mole of
react with 1 mole of 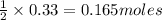 of
of