
Chemistry, 12.07.2019 02:20 Cupcake8189
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and 0.850 atm. show your work.
part 2. if this sample was placed under extremely low temperature, describe how the actual volume would compare to the predicted volume. explain your answer.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Explain why scientists use shared characteristics to make cladograms.
Answers: 1

Chemistry, 22.06.2019 15:00
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 2

Chemistry, 22.06.2019 15:30
Which of the following are correct values for the ideal gas laws constant r
Answers: 1

You know the right answer?
Part 1. determine the molar mass of a 0.622-gram sample of gas having a volume of 2.4 l at 287 k and...
Questions

Mathematics, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20





History, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20



Mathematics, 05.02.2021 19:20

History, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20

History, 05.02.2021 19:20

Mathematics, 05.02.2021 19:20


Mathematics, 05.02.2021 19:30

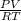
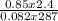
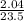 = 0.087mole
= 0.087mole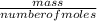
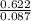 =7.15gmol⁻¹
=7.15gmol⁻¹


