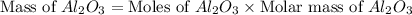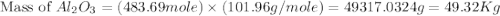Chemistry, 12.07.2019 03:20 deaerionharper
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al reacts with an excess of fe2o3,fe2o3, how many kilograms of al2o3al2o3 will be produced?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:50
Asample of tin (ii) chloride has a mass of 0.49 g. after heating, it has a mass of 0.41 g. what is the percent by mass of water in the hydrate? %
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2

Chemistry, 22.06.2019 22:30
Gusing the milligrams of ascorbic acid you entered above, the ratio of total sample volume to aliquot volume, and the total milligrams of the vitamin c tablet that you dissolved, calculate the mass of ascorbic acid in the vitamin c tablet for each trial. do this by scaling up to find the amount (mg) of ascorbic acid in your 250 ml flask. enter your calculated mass of ascorbic acid in the vitamin c tablet, for each trial. be sure to enter your calculated mass in the corresponding order that you entered your milligrams of ascorbic acid. the milligrams of ascorbic acid you entered for entry #1 previously should correspond to the mass of ascorbic acid that you enter for entry #1 here.
Answers: 1

You know the right answer?
2al(s)+fe2o3(s)−→−heatal2o3(s)+2fe( l) 2al(s)+fe2o3(s)→heatal2o3(s)+2fe(l) if 26.1 kg al26.1 kg al r...
Questions





Mathematics, 05.07.2019 04:30

Chemistry, 05.07.2019 04:40

Social Studies, 05.07.2019 04:40




Computers and Technology, 05.07.2019 04:40

Social Studies, 05.07.2019 04:40

Spanish, 05.07.2019 04:40

Mathematics, 05.07.2019 04:40


Biology, 05.07.2019 04:40

Computers and Technology, 05.07.2019 04:40

Spanish, 05.07.2019 04:40

Mathematics, 05.07.2019 04:40

Mathematics, 05.07.2019 04:40

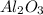 produced will be, 49.32 Kg
produced will be, 49.32 Kg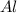 = 26.1 Kg = 26100 g
= 26.1 Kg = 26100 g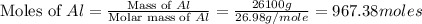
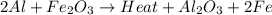
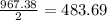 moles of
moles of